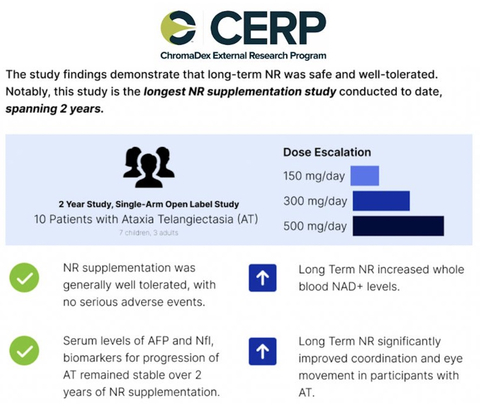
This is the longest Niagen NR clinical trial to date spanning two years and showcases that long-term Niagen NR is safe and well tolerated
ChromaDex Corp. (NASDAQ:CDXC), one of the global authorities on Nicotinamide Adenine Dinucleotide (NAD+) and healthy aging research, shares results from a phase II clinical study published in the peer-reviewed journal, Movement Disorders, by a team of researchers led by Dr. Hilde Nilsen, University of Oslo, Arkershus University Hospital, Norway. The clinical trial was part of the ChromaDex External Research Program (CERP™), which donated ChromaDex’s patented nicotinamide riboside (NR) ingredient, Niagen®, one of the most efficient and superior NAD+ precursors available, for the advancement of this research. The promising results demonstrate that long-term supplementation with Niagen NR effectively increased whole blood NAD+ levels up to fourfold, improved coordination, and enhanced eye movement while maintaining biomarkers of stable liver and kidney function in Ataxia Telangiectasia (AT) patients. AT is a rare, inherited neurodegenerative disorder characterized by premature aging, cerebellar degeneration, immunodeficiency, and cancer predisposition.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231115900463/en/
Newly published phase II clinical study demonstrates that supplementation with Niagen, patented nicotinamide riboside (NR), elevates NAD+ up to fourfold, improving motor coordination and eye movement in Ataxia Telangiectasia (AT) patients (Graphic: Business Wire)
Building on a growing body of research, this is the second published study (Veenhuis et al. in 2021) analyzing the therapeutic effects of Niagen NR supplementation in AT patients and marks a milestone as it is the longest Niagen NR clinical trial to date, spanning two years, with results showcasing that long-term supplementation is safe and well tolerated.
"NR supplementation has shown promising potential in supporting brain health and cognition,” said Dr. Andrew Shao the Vice President of Scientific & Regulatory Affairs at ChromaDex. “Independent research through CERP suggests that NR may play a role in maintaining neuronal function and promoting cognitive well-being by supporting NAD+ levels in brain cells."
Individuals diagnosed with AT frequently experience profound challenges in motor function, characterized by neurodegenerative conditions like cerebellar ataxia, resulting in impaired movement and coordination and they may exhibit oculomotor apraxia, difficulties controlling eye movements. These factors often result in a loss of independent walking ability, typically necessitating the use of a wheelchair by the age of ten.
Dr. Nilsen remarks, “While the exact mechanisms underlying cerebellar degeneration and neurodegeneration in AT are not fully understood, research indicates inability to repair and respond appropriately to DNA damage. AT research has shown that DNA damage depletes NAD+, the crucial cellular coenzyme that plays an essential role in mitochondrial function, DNA repair and cellular energy production. This growing body of research is promising as it demonstrates that NR supplementation may be a potential therapeutic strategy for AT patients.”
About the study
This two year phase II clinical study was a single-arm open-label intervention trial investigating the safety and efficacy of long-term NR supplementation in 10 patients with AT (6 were female, 4 were male, of which 7 were children, 3 were adults), between the ages of 3 to 27 years. The patients were given oral NR as an intervention starting with an initial dose of 150 mg/day, gradually increasing to 300 mg/day, and reaching a final dose of 500 mg/day.
Study highlights
- NR supplementation was well tolerated, with no serious adverse events.
- NR supplementation increased whole blood NAD+ levels up to fourfold.
- Serum levels of AFP, serum alpha-fetoprotein and Nfl, neurofilament light chain, biomarkers of AT progression, remained stable for most participants over two years of NR supplementation, suggesting that NR may slow or delay progression of AT.
-
Long-term NR supplementation reduced ataxia symptoms and improved motor coordination, eye movements, and overall neurological functioning:
- Positive correlations were observed between NR supplementation and improved AT Neurological Examination Toolkit (NEST) total score, eye movements, SARA coordination scores, ICARS coordination scores, and oculomotor scores.
- Biomarkers related to liver and kidney function did not show clinically significant changes during the two-year treatment period, indicating the NR supplementation was safe.
- Long-term blood glucose regulation, measured as HbA1c level, remained stable in all participants.
- NR supplementation did not cause changes in hematological or immunological parameters.
Relevance
Reaching a milestone in NR clinical research as the longest published clinical study to date, results of this study demonstrate that NR effectively increased whole blood NAD+ levels up to fourfold, improved coordination, and enhanced eye movement in both adults and children with AT, while maintaining biomarkers of stable liver and kidney function. The outcomes of this study strongly support the inclusion of NR supplementation as a standard clinical approach for AT, however more research needs to be conducted to confirm the impact of NR on motor skills such as standing and walking.
For additional information on the science supporting Niagen® visit www.chromadex.com.
About ChromaDex:
ChromaDex Corp. is a global bioscience company dedicated to healthy aging. The ChromaDex team, which includes world-renowned scientists, is pioneering research on nicotinamide adenine dinucleotide (NAD+), levels of which decline with age. ChromaDex is the innovator behind NAD+ precursor nicotinamide riboside (NR), commercialized as the flagship ingredient Niagen®. Nicotinamide riboside and other NAD+ precursors are protected by ChromaDex’s patent portfolio. ChromaDex maintains a website at www.chromadex.com to which ChromaDex regularly posts copies of its press releases as well as additional and financial information about the Company.
Forward-Looking Statements:
This release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934, as amended, including statements related to whether the results demonstrate that long-term supplementation with Niagen NR effectively increased whole blood NAD+ levels up to fourfold, improved coordination, and enhanced eye movement while maintaining biomarkers of stable liver and kidney function in Ataxia Telangiectasia (AT) patients, whether the results showcase that long-term supplementation is safe and well tolerated, whether NR supplementation has shown promising potential in supporting brain health and cognition, and whether this growing body of research is promising or if it demonstrates that NR supplementation may be a potential therapeutic strategy for AT patients. Statements that are not a description of historical facts constitute forward-looking statements and may often, but not always, be identified by the use of such words as "expects," "anticipates," "intends," "estimates," "plans," "potential," "possible," "probable," "believes," "seeks," "may," "will," "should," "could" or the negative of such terms or other similar expressions. Risks that contribute to the uncertain nature of these forward-looking statements include the impact of the COVID-19 pandemic on our business and the global economy; our history of operating losses and need to obtain additional financing; the growth and profitability of our product sales; our ability to maintain sales, marketing and distribution capabilities; changing consumer perceptions of our products; our reliance on a single or limited number of third-party suppliers; and the risks and uncertainties associated with our business and financial condition. More detailed information about ChromaDex and the risk factors that may affect the realization of forward-looking statements is set forth in ChromaDex's Annual Report on Form 10-K for the fiscal year ended December 31, 2021, ChromaDex's Quarterly Reports on Form 10-Q and other filings submitted by ChromaDex to the SEC, copies of which may be obtained from the SEC's website at www.sec.gov. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and actual results may differ materially from those suggested by these forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement and ChromaDex undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof.
View source version on businesswire.com: https://www.businesswire.com/news/home/20231115900463/en/
Phase II Clinical Study Demonstrates that Supplementation with Niagen®, Patented NR, Elevates NAD+ Up to Fourfold, Improving Motor Coordination and Eye Movement in AT Patients #NAD #NADSupplementation #NicotinamideRiboside
Contacts
ChromaDex Media Contact:
Kendall Knysch, Head of Public Relations & Partnerships
310-388-6706 ext. 689
kendall.knysch@chromadex.com
ChromaDex Investor Relations Contact:
+1 (949) 356-1620
InvestorRelations@ChromaDex.com
