MALVERN, Pa., June 25, 2024 (GLOBE NEWSWIRE) -- YPrime, the leading pioneer in clinical trial technology, today released a comprehensive research report titled Endocrinology Clinical Trials: Advancing Research with the Help of eCOA Technologies. The report, based on a survey of clinical trial professionals specializing in endocrinology research, emphasizes the critical role of user-centric eCOA (electronic clinical outcome assessment) technologies in addressing the unique challenges faced by the industry.
The answers to several survey questions highlight the importance of patient-centricity in endocrinology clinical trials:
- 68% of respondents cited recruitment and retention as their top concern, emphasizing the need for a patient-centric approach to encourage participation and minimize dropouts
- 46% said simplicity and user-friendliness of the interface is a top criterion in selecting an eCOA solution
- 54% stated that a key benefit of eCOA is its ability to collect vital patient-reported outcomes
- 52% said it offers patient quality-of-life measures, underlining eCOA's critical role in capturing patient-centric data
"At YPrime, we understand that every therapeutic area has its own unique challenges and requirements," said Mike Hughes, Chief Product Officer at YPrime. "By closely collaborating with patients, site staff, and sponsors, we develop eCOA solutions tailored to the specific needs of endocrinology trials. Our user-centric approach ensures that our technologies are not only cutting-edge but also intuitive and easy to use, ultimately leading to better patient engagement, higher data quality, and faster study timelines."
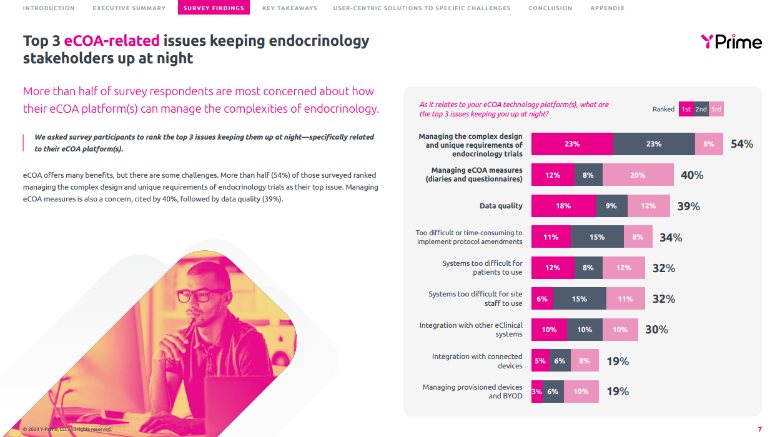
The report also highlights the growing adoption of connected devices in endocrinology clinical trials, with 44% of respondents already leveraging these technologies, primarily for at-home patient monitoring – and another 46% considering adoption. In related news, YPrime recently announced the launch of its groundbreaking glucometer functionality, which integrates seamlessly with its eCOA platform. Developed in close collaboration with patients living with diabetes, this innovative feature promises to transform clinical trials with blood glucose endpoints by delivering a patient-centric, intuitive, and connected experience.
Please visit the YPrime website for the full version of Endocrinology Clinical Trials: Advancing Research with the Help of eCOA Technologies. The report offers valuable insights and practical recommendations for clinical trial professionals looking to optimize their endocrinology studies with the help of eCOA technologies.
About YPrime
At YPrime, we streamline the clinical trial journey with a configurable platform designed for speed, quality, and certainty. With 50% faster IRT startup times, up to 30% faster eCOA launch times, and quality standards 50% above the industry average, YPrime can help you solve for certainty. Discover how by visiting www.yprime.com or emailing marketing@yprime.com.
Media Contact
Terry Rehm
Head of Thought Leadership and Public Relations, YPrime
trehm@yprime.com
862-288-0329
An infographic accompanying this announcement is available at: https://www.globenewswire.com/NewsRoom/AttachmentNg/3a9e66f2-0df1-4529-a9b4-91994c1fda44

