Clinical data builds upon body of evidence suggesting EXS-21546 is a highly potent and selective A2AR antagonist with low CNS exposure
Exscientia anticipates initiating Phase 1b/2 in patients with high adenosine signature cancers in second half of 2022
Ongoing translational work to establish predictive biomarker to enable targeting of patients most likely to benefit from EXS-21546
Exscientia plc (Nasdaq: EXAI) today announced data from its Phase 1 healthy volunteer study of EXS-21546, its highly selective A2A receptor antagonist co-invented and developed through a collaboration between Exscientia and Evotec SE (Frankfurt Stock Exchange: EVT, MDAX/TecDAX, ISIN: DE0005664809; Nasdaq: EVO). Topline data from this healthy volunteer study confirmed Exscientia’s target product profile design, including potency, high receptor selectivity and expected low brain exposure with no CNS adverse events reported, supporting advancement of EXS-21546 to a Phase 1b/2 study in patients with solid tumours exhibiting high adenosine signatures.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220614005457/en/
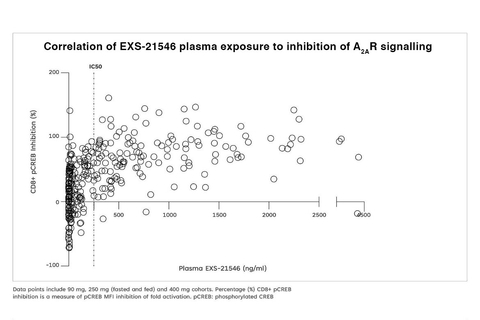
(Graphic: Business Wire)
“Topline data from our phase 1a study of EXS-21546 demonstrate the ability of our AI-based platform to create novel molecules based upon defined design objectives and with a high level of translatability to human biology. EXS-21546 is a pilot programme from the early days of our platform, and we are proud that it achieved our targeted objectives of potency, selectivity and pharmacokinetics,” said David Hallett, Ph.D., Chief Operating Officer and head of drug discovery for Exscientia. “Moving forward, a primary challenge in clinical development of an A2AR antagonist is identifying patients who will benefit the most from this type of immunomodulatory therapy. We believe that utilising our unique precision medicine platform to analyse patient tumour microenvironments ex vivo, including immune function, will help us identify the right patients for our drug.”
The EXS-21546 phase 1a study was a three-part dose-finding trial evaluating the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of single ascending doses (SAD) and multiple ascending doses (MAD) of EXS-21546. The study randomised 60 healthy male subjects across all three parts. One of the study’s primary goals was to inform the optimal starting dose for EXS-21546 for the Phase 1b/2 study in cancer patients.
The study showed that observed human PK for EXS-21546 was in line with what had been designed for and predicted in preclinical modeling, supporting a twice-daily (BID) dose for continuous A2A receptor inhibition over a dosing interval.
EXS-21546 showed dose-dependent inhibition of CREB phosphorylation in CD8-positive cells, with the PD profile mirroring plasma exposure. Inhibition of A2A receptor signaling was sustained over the BID dosing period, demonstrating a level of lasting target engagement.
EXS-21546 was well-tolerated with no CNS adverse events reported in the SAD portion at all doses (30mg, 90mg, 250mg, 400mg) and in the MAD portion at 150mg BID. In the MAD phase, a lab abnormality of elevated ALT and AST was observed in one subject that was classified as a Grade 3 Serious Adverse Event. This event was observed in one subject three days after completion of 14 days of treatment and resolved without medical intervention. The subject was asymptomatic during the treatment period and reported no adverse events while on drug.
Based on these results, Exscientia expects to initiate a Phase 1b/2 study of EXS-21546 in patients with high adenosine signature solid tumours in the second half of 2022. The Phase 1b/2 study is being designed to evaluate higher doses of EXS-21546.
The Company expects to share additional data from this Phase 1a study at future medical meetings.
About EXS-21546
EXS-21546, an AI-designed A2A receptor antagonist, was co-invented and developed by Exscientia and Evotec. Exscientia designed the compound leveraging its AI-driven platform and Evotec provided biology and chemistry capabilities.
Some tumours produce high levels of adenosine, which binds and activates A2A receptors on immune cells, resulting in the suppression of the anti-tumour activity of the immune system. EXS-21546 is being investigated for its ability to prevent high concentrations of adenosine from activating the A2A receptor, and thereby its potential to promote the anti-tumour activity of the immune cells.
About the EXS-21546 Phase 1a Trial
The Phase 1 study was a three-part study in male healthy volunteers to assess the safety, tolerability, pharmacokinetics and pharmacodynamics (PK/PD) of EXS-21546. Part 1 was a randomised, double-blind, placebo-controlled, SAD study with a food effect assessment where 41 healthy volunteers were randomised 3:1 (in a ratio of 6 active to 2 placebo per cohort). Part 2 was a randomised, double-blind, placebo controlled, MAD study over 14 days. Part 2 was completed after the enrolment of 1 cohort (8 subjects) who received 150mg EXS-21546 BID. Part 3 was a 3-way crossover, open label, randomised study, where 11 subjects were enrolled to evaluate a capsule formulation (fed and fasted) as compared to an oral suspension (fasted) formulation.
About Exscientia
Exscientia is an AI-driven pharmatech company committed to discovering, designing and developing the best possible drugs in the fastest and most effective manner. Exscientia developed the first-ever functional precision oncology platform to successfully guide treatment selection and improve patient outcomes in a prospective interventional clinical study, as well as to progress AI-designed small molecules into the clinical setting. Our pipeline demonstrates our ability to rapidly translate scientific concepts into precision-designed therapeutic candidates, with more than 30 projects underway. By designing better drugs, faster, we believe the best ideas of science can rapidly become the best medicines for patients.
Exscientia is headquartered in Oxford (England, U.K.), with offices in Vienna (Austria), Dundee (Scotland, U.K.), Boston (Mass., U.S.), Miami (Fla., U.S.), Cambridge (England, U.K.), and Osaka (Japan).
Visit us at https://www.exscientia.ai or follow us on Twitter @exscientiaAI.
Forward-Looking Statements
This press release contains certain forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including statements with regard to Exscientia’s expectations with respect to the progress of development of candidate molecules, timing and progress of, and data reported from, preclinical studies and clinical trials of Exscientia’s product candidates, and Exscientia’s expectations regarding its precision medicine platform and AI-driven drug discovery platform. Words such as “anticipates,” "believes," “expects,” "intends," "projects," "anticipates," and "future" or similar expressions are intended to identify forward-looking statements. These forward-looking statements are subject to the uncertainties inherent in predicting future results and conditions, including the scope, progress and expansion of Exscientia’s product development efforts; the initiation, scope and progress of Exscientia’s and its partners’ clinical trials and ramifications for the cost thereof; clinical, scientific, regulatory and technical developments; and those inherent in the process of discovering, developing and commercialising product candidates that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such product candidates. Exscientia undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220614005457/en/
Contacts
Investors:
Sara Sherman
investors@exscientia.ai
Media:
Amanda Galgay
media@exscientia.ai

