Creative Diagnostics, a reagent supplier and developer focused on biologics quality control, is pleased to announce the launch of its comprehensive suite of Host Cell DNA Assay Kits. These innovative kits empower researchers and manufacturers to effectively detect and quantify residual host cell DNA impurities in biological products, ensuring product safety and regulatory compliance throughout the biopharmaceutical manufacturing process.
The detection of residual host cell DNA throughout the biopharmaceutical manufacturing process is an important step in ensuring product safety and batch validation. As an expert in bioprocess impurity analysis, Creative Diagnostics offers host cell DNA assay kits for the detection of host cell DNA impurities in specific recombinant expression systems in biologic products, targeting mammalian, yeast, bacterial, viral, insect cells and more. These products can be used in biological agent development to provide researchers with host cell DNA information to guide bioprocess optimization and reduce host cell DNA levels.
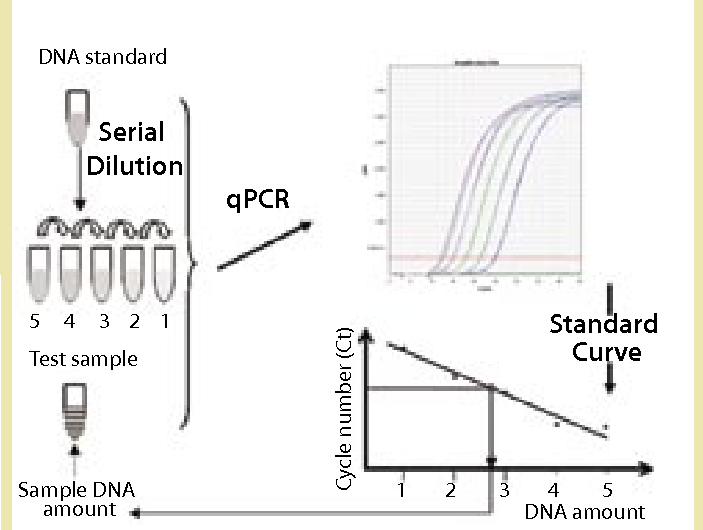
Creative Diagnostics provides host cell DNA assay kits for specific cell line expression systems based on the qPCR technique, which can be used to rapidly detect residual host cell DNA contamination in biologics at any stage of product development. Biological products (e.g., recombinant proteins, antibodies, and vaccines) are expressed from host cells such as bacteria, yeast, animal cells and continuous cell lines during the production process. Even after a rigorous purification process, the products still contain fragments of DNA from the host cells.
In addition, Creative Diagnostics assay kits provide highly sensitive and accurate detection and quantification of host cell DNA impurities. The residual DNA molecules present in the human body with biological products can lead to an increased risk of carcinogenesis, infection and immunomodulation. The WHO and the European Union allow residual DNA levels of up to 10 ng/dose, and the US FDA allows such levels of up to 100 pg/dose. To meet these requirements, highly sensitive and accurate methods are needed to detect and quantify low levels of DNA.
For example, the E. coli DNA Residue Assay Kit (Cat. No. DDNA-021) is based on the principle of the PCR fluorescent probe method for the quantitative detection of E. coli DNA residues in samples, which is fast, specific and reliable, with the lowest detection limit of fg level. The kit is supplied with a reference standard for the quantification of E. coli DNA, and can be used in conjunction with the Creative Diagnostics Host Cell DNA Prep Kit to accurately determine the amount of residual E. coli DNA in biological samples.
Creative Diagnostics' host cell DNA assay kits are designed to detect impurities in recombinant expression systems and include a wide range of required assays. The company also offers program-specific host cell DNA assay kit development services. For more information, please visit https://qbd.creative-diagnostics.com/products/host-cell-dna-assay-kits.html.
About Creative Diagnostics
Creative Diagnostics is a global leader in the development and manufacturing of innovative tools and reagents for bioprocess impurity analysis. The company offers a comprehensive portfolio of solutions to support researchers in the quality control of biologics and provides biopharmaceutical quality, purity and safety assays, analytical methods and applications for the biotechnology and biopharmaceutical industries.
Media Contact
Company Name: Creative Diagnostics
Contact Person: Thomas Schmitt
Email: Send Email
State: New York
Country: United States
Website: https://qbd.creative-diagnostics.com/